Saving the Ozone Layer
Wednesday July 19, 2023



Life as we know it would not exist without the sun. Its light gives us warmth, energy, and guides the biological processes that make our planet habitable. However, too much of the sun’s energy, specifically in the form of ultraviolet (UV) radiation, can be harmful to life on Earth. This is where the ozone layer comes into play.
The ozone layer, a relatively thin sheet of ozone molecules located in the stratosphere about 10 to 30 kilometers above Earth’s surface, plays a vital role in protecting us from the sun’s harmful UV radiation. This layer absorbs most of the sun’s harmful UVB radiation, which if not blocked, can cause skin cancer and cataracts in humans, harm aquatic ecosystems, and disrupt plant growth.
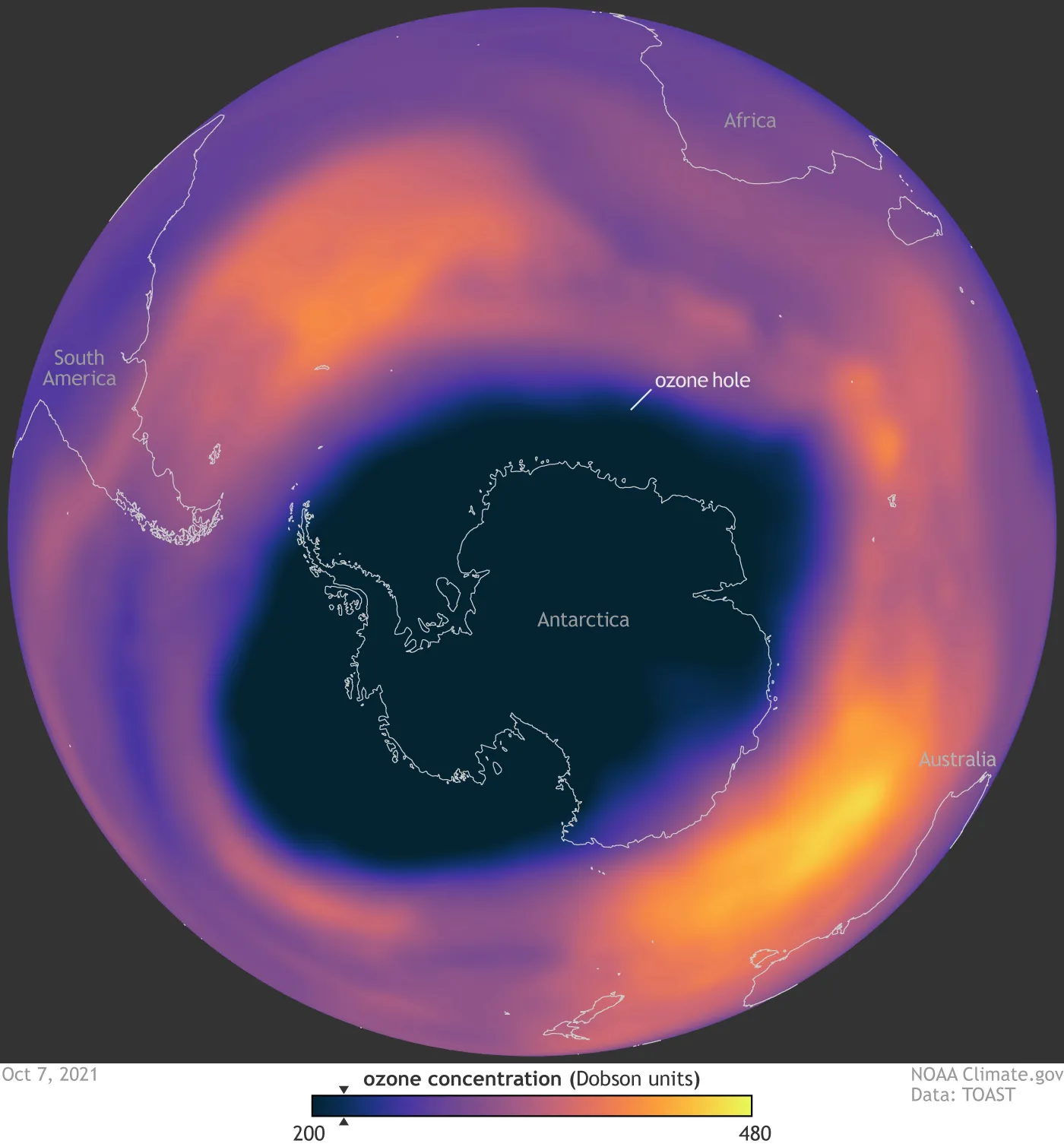
However, in the mid-20th century, scientists began to notice a disturbing trend. The ozone layer was thinning, and in some places, particularly over Antarctica, large ‘holes’ were appearing. This phenomenon, known as ozone depletion, was a serious concern because it threatened to let more UV radiation reach the Earth’s surface, posing a significant risk to the biosphere.
Investigations into the cause of this depletion led scientists to identify a group of human-made chemicals known as ozone-depleting substances (ODS). Used widely in air conditioning, refrigeration, aerosols, and as cleaning processes for electronic equipment, ODS were being released into the atmosphere in significant quantities. Once in the atmosphere, these substances could stay for a long time, slowly rise to the stratosphere, and through a series of chemical reactions, destroy the ozone layer.
The discovery of this potentially catastrophic environmental issue prompted the world to take action. As a result, nations came together in an unprecedented show of global unity to address this threat, resulting in the Montreal Protocol.
The Montreal Protocol on Substances that Deplete the Ozone Layer is an international treaty designed to protect the ozone layer by phasing out the production and consumption of numerous substances that are responsible for ozone depletion. Since its adoption in 1987, the Montreal Protocol has been ratified by 197 countries - making it one of the most universally ratified treaties in United Nations history.
This post will dive deep into the issues surrounding ozone depletion, focusing on common misconceptions, the science of ozone-depleting substances, their origins, the triumph of the Montreal Protocol, and the current state of our ozone layer. We will also discuss the further steps we should take to ensure its full recovery.
It’s often the case that when significant scientific issues enter the public sphere, they bring with them a wave of misinformation and misunderstanding. The issue of ozone depletion is no exception. Despite the overwhelming scientific consensus, some individuals and groups have claimed that the “ozone hole” is a myth, a concocted scare story that never came to fruition. They point to predictions made decades ago that warned of severe consequences such as increased cancer rates and environmental damage, which they argue have not been realized to the predicted extent.

To fully comprehend these misconceptions, it’s necessary to delve into the scientific process that led to the early warnings about ozone depletion. In the 1970s and 1980s, scientists began making projections about the future state of the ozone layer. They warned that if the production and use of ODS were not curtailed, significant thinning of the ozone layer could occur, leading to what is often referred to as an “ozone hole.” This hole, they projected, would increase the Earth’s exposure to harmful UVB radiation with severe health and environmental implications.
It’s important to understand that these were not wild guesses but informed predictions based on established scientific principles and observed trends. However, like any scientific model, they were subject to a degree of uncertainty, dependent on various factors such as future ODS emissions, technological changes, policy responses, and natural variability.
These predictions proved alarmingly accurate. Measurements have shown a significant thinning of the ozone layer over the last few decades, especially over Antarctica where an actual “ozone hole” forms each spring. However, the direst predictions didn’t materialize as projected, not because they were fundamentally flawed, but primarily because the world took them seriously and acted.
The Montreal Protocol, the very subject of this discussion, is a testament to that action. It was a landmark agreement that led to a drastic reduction in the global production and consumption of ODS. Without the Protocol, and the scientific predictions that precipitated it, the world might have experienced far more severe ozone depletion and its harmful consequences.
Moreover, it is crucial to appreciate the dynamic nature of Earth’s atmosphere and the intricate processes that govern the ozone layer. While the term “ozone hole” is a popular simplification, it can be misleading. The phenomena we are discussing involve complex changes to the distribution and concentration of ozone in the stratosphere, influenced by a multitude of factors such as temperature, wind patterns, and solar radiation.
The dismissal of ozone depletion as a myth ignores these complexities and the significant scientific evidence that supports this phenomenon. The decreases in stratospheric ozone have been well documented, as have the increases in harmful UVB radiation in areas of ozone thinning. This evidence is a product of decades of research, involving countless scientists and extensive international collaboration.
Scientists use a variety of sophisticated methods to study the ozone layer, from ground-based monitoring stations to high-altitude balloons and satellites. These tools have provided a wealth of data, painting a clear picture of the changes to the ozone layer and affirming that ozone depletion is not a myth but a real and pressing issue.
While it’s true that the situation has improved since the darkest predictions were made, this is not a cause for complacency. Ozone depletion has been curbed but not eliminated. The fight is not over yet. The ozone layer’s full recovery is still decades away, and its protection remains as important today as it was when the issue first came to light.
In the following sections, we will further unravel this issue, exploring the science of ozone-depleting substances, their origins, and the remarkable story of how the world came together to address this global threat.
To fully appreciate the issue of ozone depletion, it’s crucial to understand the villains of the story - the ozone-depleting substances (ODS). These are a group of man-made chemicals that have the potential to reach the stratosphere and, once there, destroy the ozone layer. But what makes these substances so destructive to the ozone layer, and how exactly do they operate?
The primary culprits in ozone depletion are a class of chemicals known as halocarbons, specifically chlorofluorocarbons (CFCs), halons, carbon tetrachloride, and methyl chloroform. Halocarbons contain different combinations of carbon with elements such as chlorine, fluorine, and bromine. What makes these chemicals particularly harmful to the ozone layer is their stability, longevity, and the presence of chlorine and bromine.
ODS are incredibly stable, which allows them to remain in the atmosphere for a long time after being released - some can persist for more than a century. This longevity gives them ample time to gradually rise into the stratosphere. Once in the stratosphere, these substances can be broken down by solar radiation, releasing chlorine or bromine atoms.
Here’s where the destructive process begins. A single chlorine atom can catalyze the destruction of many thousands of ozone molecules before it is removed from the stratosphere. This reaction is facilitated by the intense sunlight and cold conditions found particularly in the polar regions. Bromine, while less common, is even more efficient at destroying ozone.
This process is not an immediate one, which adds to the insidious nature of ODS. The effects of these chemicals on the ozone layer are delayed, meaning the full impact of substances released today might not be felt for several decades. However, once the damage is done, it also takes a long time to recover. Even with the substantial reductions in ODS emissions achieved through the Montreal Protocol, it is expected to take many decades for the ozone layer to fully recover.
The role of ODS in ozone depletion is more than just a theory. It’s a well-established scientific fact, backed up by a vast body of research. Observations have shown clear correlations between the presence of these substances in the stratosphere and decreases in ozone. Moreover, the most significant reductions in ozone have been observed over Antarctica, where conditions are most conducive to the chemical reactions that destroy ozone.
Understanding the science of ODS and their impact on the ozone layer was a critical first step in addressing the problem of ozone depletion. It highlighted the need for action and guided the development of policies aimed at reducing the production and use of these harmful substances. However, science alone could not solve the problem. It was also necessary to understand where these substances were coming from and why they were being used. In the next section, we’ll delve into the origins of ODS and the challenges faced in reducing their use.
The origins of ozone-depleting substances (ODS) are deeply intertwined with human industrial and technological development throughout the 20th century. These substances, remarkable for their stability and versatility, found widespread application in various sectors, contributing significantly to modern comforts and conveniences. Unfortunately, the environmental consequences of their use went unnoticed for decades.
Chlorofluorocarbons (CFCs), one of the most notorious types of ODS, were first developed in the 1920s and 1930s. They emerged as a safer alternative to the dangerous chemicals then used in refrigeration, such as ammonia, sulfur dioxide, and propane. CFCs were non-toxic, non-flammable, and highly stable, making them an ideal choice for various applications. They quickly found their way into air conditioners, refrigerators, aerosol propellants, and later, as blowing agents for foams and insulation materials.
Other ODS, such as carbon tetrachloride and methyl chloroform, were widely used in industrial processes. Carbon tetrachloride was a common solvent and cleaning agent, especially in the electronics industry for cleaning circuit boards. Methyl chloroform was used as an industrial solvent for cleaning and degreasing, as well as in some aerosol products.

Halons, another group of ODS, were primarily used in fire extinguishers, especially for high-risk environments like airplanes and data centers. They were favored because they could rapidly extinguish fires without leaving residue that could damage sensitive equipment.
In all these applications, the valuable properties of ODS—stability, non-reactivity, and efficiency—were a double-edged sword. While they made ODS suitable for their respective applications, they also meant these substances could survive in the environment for a long time and eventually reach the ozone layer.
The production and consumption of ODS grew rapidly throughout much of the 20th century, reaching a peak in the late 1980s. By the time the ozone-depleting properties of these substances were discovered, they were deeply ingrained in global industrial processes and everyday consumer products. This presented a significant challenge: how could these substances be phased out without disrupting critical industries and services?
It was against this backdrop that the Montreal Protocol was negotiated. The treaty had to address not only the environmental and scientific aspects of ozone depletion but also the economic, industrial, and societal implications of phasing out ODS. The success of the Protocol in achieving this delicate balance is a testament to the power of international cooperation and a model for addressing other global environmental challenges.
However, the story of ODS is not entirely a thing of the past. Despite the success of the Montreal Protocol, these substances still pose a threat to the ozone layer today. Illegal production and use, improper disposal of old equipment, and the long lifetime of these substances in the environment all contribute to ongoing ozone depletion. In the subsequent sections, we will explore the impact of the Montreal Protocol and the ongoing challenges in ensuring the recovery of the ozone layer.
The Montreal Protocol, formally known as the Montreal Protocol on Substances That Deplete the Ozone Layer, stands as a beacon of successful international environmental cooperation. The treaty, which came into effect in 1989, brought together nations from around the world with a common aim: to protect the ozone layer by phasing out the production and use of ozone-depleting substances (ODS).
The idea of global collaboration to address ozone depletion was not new; it had been proposed and discussed in various scientific and policy circles. However, it was the discovery of the Antarctic ozone hole in the mid-1980s that injected a sense of urgency into these discussions. This dramatic finding, combined with the growing body of evidence linking ODS to ozone depletion, brought the issue to the forefront of global attention.
The Montreal Protocol was not an instant fix but rather an evolving agreement. It included provisions for adjustment and amendment in light of new scientific understanding and technological advancements. This flexibility has been a key factor in its effectiveness. As knowledge about the ozone layer grew and as alternatives to ODS became more viable, the Protocol was amended and adjusted to accelerate the phase-out of these substances.
Perhaps the most significant demonstration of the Protocol’s adaptability was the 1990 London Amendment. This amendment established the Multilateral Fund, which assists developing countries in meeting their Protocol obligations. Recognizing that developing countries might struggle to replace ODS with more expensive alternatives, the fund has provided financial assistance for transition efforts, fostering a more equitable approach to the global challenge of ozone protection.
The Montreal Protocol has been remarkably successful in achieving its objectives. Since its implementation, the global consumption of ODS has decreased by over 98%. This reduction has significantly slowed the rate of ozone depletion and set the stage for the eventual recovery of the ozone layer. It’s estimated that without the Protocol, the levels of ozone-depleting substances in the atmosphere could have been ten times higher by the middle of the 21st century, leading to significantly more ozone depletion and associated health and environmental problems.
Moreover, the Protocol has had unexpected benefits in combating climate change. Many ODS are potent greenhouse gases, with much higher warming potentials than carbon dioxide. The reduction in ODS emissions due to the Protocol has therefore significantly reduced global warming. In fact, it’s estimated that the Montreal Protocol has delayed the progression of climate change by up to a decade.
The Montreal Protocol serves as a compelling model of how international cooperation can address global environmental challenges. It showcases how scientific understanding, coupled with political will and public support, can drive effective action. It highlights the importance of flexibility, allowing agreements to evolve with new knowledge and circumstances. And it underscores the need for fairness, assisting those who may struggle more with the transition.
However, the Protocol is not a panacea, and the work is far from over. The long lifetimes of ODS mean that they will continue to affect the ozone layer for many years to come. Additionally, some challenges have emerged, such as the unexpected rise in emissions of CFC-11, a banned substance, detected in recent years. Addressing these ongoing and emerging challenges is crucial to ensuring the full recovery of the ozone layer, a topic we will delve into in the following section.
In the decades since the enactment of the Montreal Protocol, there has been notable progress in the restoration of the ozone layer. Due to the significant reduction in the production and release of ozone-depleting substances (ODS), we have moved from an era of rapid ozone layer depletion to one of potential recovery.
Reports from the World Meteorological Organization (WMO) and the United Nations Environment Programme (UNEP) suggest that the ozone layer is showing signs of healing. Between 2000 and 2019, parts of the ozone layer have recovered at a rate of 1-3% per decade. If this trend continues, the ozone layer over the northern hemisphere and mid-latitude regions is projected to heal completely by the 2030s, followed by the southern hemisphere in the 2050s and the polar regions by 2060.
A significant indicator of this recovery is the gradual decrease in the size of the Antarctic ozone hole. Observations show that the hole is becoming smaller and less deep. The year 2019 saw the smallest ozone hole since its discovery, an encouraging sign, although it is crucial to remember that annual variations are influenced by weather patterns as well as the decrease in ODS.
Despite this promising progress, the road to full recovery is a long one. The very properties that made ODS so useful—stability and longevity—mean that they linger in the atmosphere for a long time. Even with no further emissions, it will take many decades for the ozone layer to recover fully.
Furthermore, the story of ozone layer recovery is not straightforward. Recent reports have raised concerns about unexpected emissions of some banned ODS, notably CFC-11. Studies suggest that these emissions are coming from new, illegal production, highlighting the importance of continued monitoring and enforcement of the Montreal Protocol.
Moreover, emerging research indicates potential threats from certain short-lived substances, like dichloromethane, which can deplete the ozone layer. These substances are not currently regulated by the Montreal Protocol as they were thought to break down in the atmosphere before they could reach the stratosphere. However, recent observations show increasing concentrations of these substances in the atmosphere, calling for further investigation.
Climate change also adds a layer of complexity to the issue. Changes in temperature and atmospheric circulation patterns can influence ozone concentrations. There is still much we don’t understand about these interactions and their potential implications for the ozone layer.
In the next and final section, we will explore the ongoing efforts required to ensure the full recovery of the ozone layer and preserve it for future generations.
While the progress made under the Montreal Protocol is undeniably promising, the full recovery of the ozone layer requires continued commitment and action. The journey is far from over. The long lifetimes of ozone-depleting substances (ODS), ongoing illegal activities, emerging threats, and the interplay between ozone depletion and climate change present challenges that demand our ongoing vigilance and proactive efforts.
To ensure the full healing of the ozone layer, it is essential to maintain strict adherence to the Montreal Protocol. Compliance must not be taken for granted. The unexpected rise in CFC-11 emissions, likely from new, illegal production, underscores the need for robust monitoring and enforcement mechanisms. This includes not only monitoring atmospheric concentrations of ODS but also tracking production and trade activities and strengthening compliance and enforcement mechanisms.
At the same time, it’s crucial to address the challenges posed by substances not currently controlled by the Montreal Protocol. Certain short-lived substances, like dichloromethane, are showing increasing atmospheric concentrations. Research is needed to understand their potential impact on the ozone layer and evaluate whether additional control measures are required.
Meanwhile, as we phase out ODS, we need to ensure that their replacements do not cause other environmental problems. Many ODS have been replaced by hydrofluorocarbons (HFCs), which do not harm the ozone layer but are potent greenhouse gases. Under the Kigali Amendment to the Montreal Protocol, countries have agreed to phase down the use of HFCs, aiming to prevent a significant increase in global warming.
The interplay between ozone depletion and climate change also requires further investigation. Changes in temperature and atmospheric circulation patterns can influence the distribution and concentration of ozone. We need to improve our understanding of these complex interactions to effectively protect the ozone layer in a changing climate.
Moreover, the phase-out of ODS provides an opportunity to promote energy efficiency. For instance, as we transition away from HFCs in cooling equipment, we can also improve the energy efficiency of these devices, achieving significant climate benefits.
Education and awareness-raising are another vital part of the puzzle. Many people are not aware of the issues surrounding the ozone layer and the Montreal Protocol’s success. Increasing public awareness can foster support for the actions needed to protect the ozone layer and inspire action on other environmental issues.
The healing of the ozone layer is a long-term project, one that requires the ongoing commitment of all nations. The Montreal Protocol has set us on the right path, but the journey is not over. By staying the course and addressing emerging challenges, we can ensure a safe, stable ozone layer for future generations – a testament to what we can achieve through global cooperation and scientific understanding.
The story of the ozone layer and the Montreal Protocol is one of humanity’s greatest environmental success stories. It is a testament to what can be achieved when nations come together, driven by scientific understanding and a common purpose, to confront a global challenge.
Over the past few decades, through international collaboration under the Montreal Protocol, we have made significant strides in reversing the damage to the ozone layer. The Protocol’s adaptability, its focus on fairness, and its commitment to science have been vital in this success. Its impact extends beyond ozone protection, also contributing to the fight against climate change due to the dual role many ozone-depleting substances play as potent greenhouse gases.
However, this narrative is not yet complete. While we are on a path towards the recovery of the ozone layer, this journey is far from over. The long-lasting nature of ozone-depleting substances, continued illegal activities, the emergence of new potential threats, and the complexity introduced by climate change all require our sustained attention and effort.
The future of the ozone layer depends on our continued commitment to the principles and actions embodied in the Montreal Protocol. As we move forward, we must uphold its spirit of cooperation, remain guided by science, and tackle emerging challenges with resolve.
As we look towards a future where the ozone layer can fully recover, let’s also remember the lessons the Montreal Protocol offers us. Its success serves as a beacon of hope, a testament to what we can achieve, and a model to emulate as we confront other pressing global environmental challenges.